CHEMICAL KINETICS
CHEMICAL KINETICS
Chemical kinetics, or reaction kinetics, is a branch of physical chemistry concerned with understanding the rates of chemical reactions.
Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction, and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction.
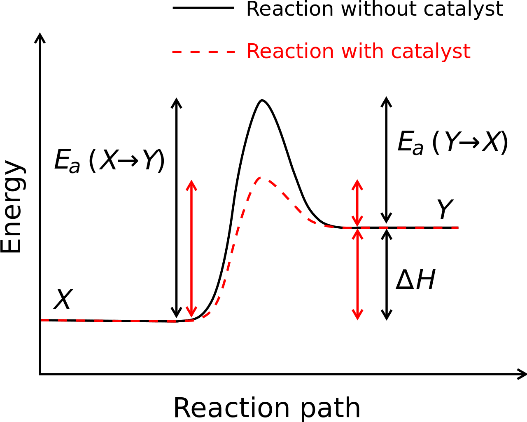
Generic potential energy diagram showing the effect of a catalyst in a hypothetical endothermic chemical reaction. The presence of the catalyst opens a new reaction pathway (shown in red) with a lower activation energy. The final result and the overall thermodynamics are the same.
FACSIMILE’s mathematical models provide chemists and chemical engineers with tools to better understand and describe chemical processes such as food decomposition, microorganism growth, stratospheric ozone decomposition, and the chemistry of biological systems. These models can also be used in the design or modification of chemical reactors to optimise product yield, more efficiently separate products, and eliminate environmentally harmful by-products.
When performing catalytic cracking of heavy hydrocarbons into gasoline and light gas, for example, kinetic models can be used to find the temperature and pressure at which the highest yield of heavy hydrocarbons into gasoline will occur.
For more direct examples of chemical kinetic applications for FACSIMLE, follow the FACSIMLE Applications links: